General Terms in Thermodynamics
General Terms in Thermodynamics: Overview
This topic covers concepts, such as, Thermodynamic System,System, Surroundings and Boundaries,General Terms in Thermodynamics etc.
Important Questions on General Terms in Thermodynamics
Time taken to heat water upto a temperature of (from room temperature) is and time taken to heat mustard oil (of same mass and at room temperature) upto a temperature of is , then (given mustard oil has smaller heat capacity)
For the process to occur under adiabatic conditions, the correct condition is:
Which is not the microscopic variable of the system?
A system is said to be an isolated system if
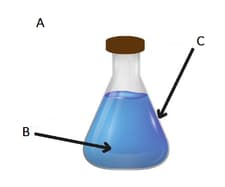
If we want to study the liquid inside the flask, we can define A, B and C as
What is a boundary for a thermodynamic system?
For an isolated system, the entropy:
Which among this is not an exothermic reaction?
What is the equation of entropy?
Entropy occurs due to ______.
An increase in enthalpy leads to an increase in _______.
Which of the following is/are macroscopic variables:
An isolated system exchanges energy and matter with surroundings.
What is an isolated system?
Which of the following is/are macroscopic variables:
Macroscopic variable is a measurable quantity used to describe the complete _____ of the system.
The variables like pressure, volume, temperature of a thermodynamic system are microscopic.
Which of the following is/are macroscopic variables:
An open system is one in which
Which of the following parameters can define the thermodynamic state of a system?
